With the popularity of electric vehicles around the world and the explosion of energy storage demand, the shortage of battery resources has become the latest problem. Many people are excited about the emergence of sodium-ion batteries, as if the new battery idea of "using salt instead of rare metals" can greatly solve the fundamental problems of low cost and resource scarcity.
Sodium-ion batteries do have the advantages of "sufficient reserves and affordable prices". After all, compared with lithium, sodium resources are abundant and cheap. Sodium batteries do not need to use "precious metals", and the cost is reduced by more than 30%. According to the above simple and simplistic ideas, it seems that sodium-ion batteries are the chosen ones of batteries and will definitely be the main development direction in the future.
Since it is such an excellent battery material, why is sodium ion not popularized? We conduct a comprehensive comparison as follows:
1. Similarities
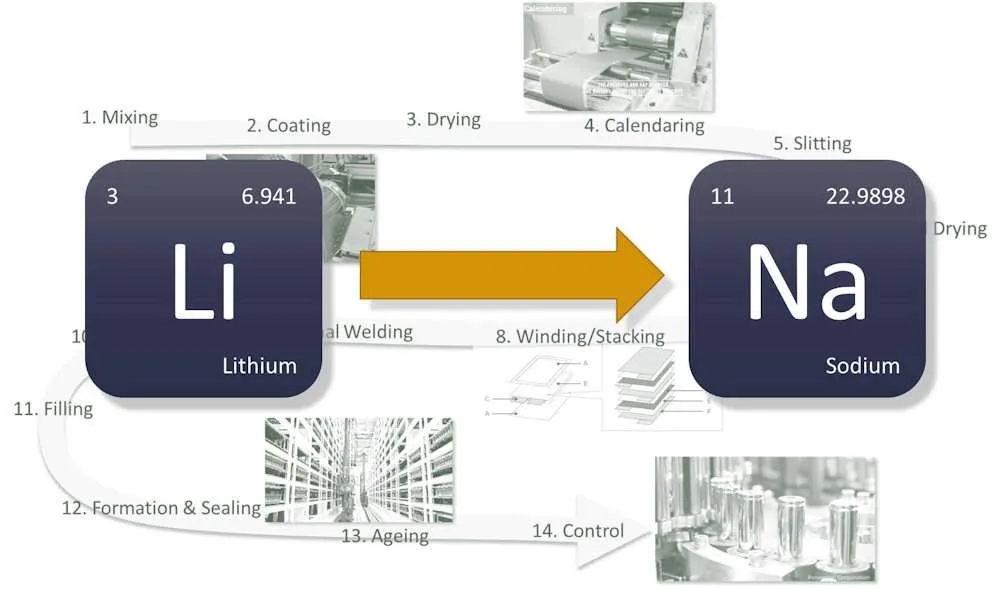
Lithium-ion batteries and sodium-ion batteries have the same working principle and battery structure. From a chemical point of view, sodium and lithium belong to the same main group in the periodic table, and their physical and chemical properties are similar. Therefore, the charging and discharging process of the two is the same. During the charging process, metal ions are released from the positive electrode material and embedded in the negative electrode material through the electrolyte and the separator, while electrons flow from the positive electrode to the negative electrode through the external circuit. The discharge process is exactly the opposite. Similarly, from the perspective of battery structure, both are composed of a positive electrode, a negative electrode, a separator, an electrolyte, and a current collector.
2. Differences
The advantages of sodium batteries are reflected in cost, low-temperature performance and safety. First, the cost of battery materials is low. The earth is rich in sodium resources, with an element content of about 23,000 ppm (while lithium is only 17 ppm), and the reserves in the earth's crust are as high as 2.64%. In addition, the positive and negative fluids of the battery can both use low-cost aluminum foil, so the mass production cost is about 35% lower than that of lithium-ion batteries. Second, the discharge performance is excellent in low-temperature environments. At -20°C, the capacity retention rate is close to 90%, and it can work normally in the temperature range of -40°C to 80°C. Third, the battery is highly safe. It does not catch fire or explode in battery safety test items such as puncture, extrusion, overcharging, and over-discharging.
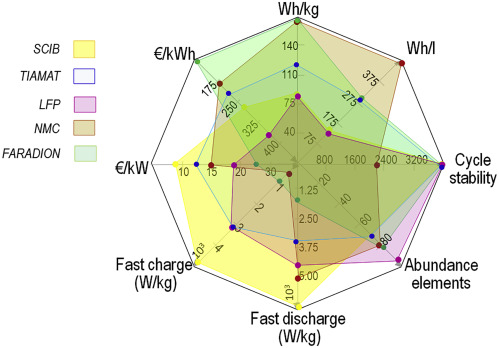
The disadvantage of sodium batteries is reflected in energy density. The radius of sodium ions is larger than that of lithium ions, and the number of ions contained per unit volume is less, resulting in fewer electrons that can be transferred during the charge and discharge process. Macroscopically, the storage capacity is lower than that of lithium-ion batteries. At present, the energy density of sodium batteries is in the range of 90-160Wh/Kg, and the theoretical range does not exceed 400 kilometers, which is far less than that of lithium batteries. This is a hard flaw of sodium batteries, which cannot meet the needs of most new energy vehicles and can only hand over the market to lithium batteries.

3. Conclusion
In general, sodium batteries and lithium batteries have their own advantages and disadvantages, and each has its own areas of expertise. Sodium batteries are more competitive in terms of cost, low-temperature performance and safety. Although it is currently in the early stages of industrialization, the potential market is huge. As the application scenarios continue to expand, sodium batteries will eventually "stand out".





 português
português  English
English français
français русский
русский español
español português
português العربية
العربية 日本語
日本語 Melayu
Melayu Indonesia
Indonesia 中文
中文




 rede suportada
rede suportada